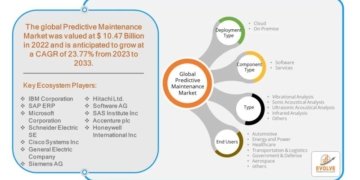
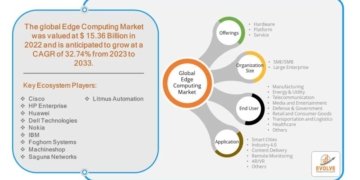
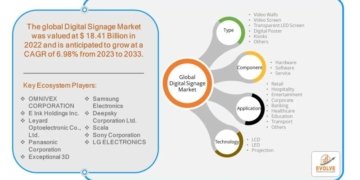
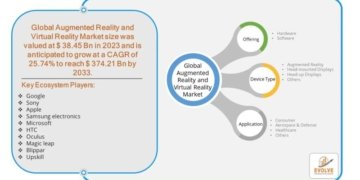
- FDA’s approval of YUTREPIA paves the way for prescribers to add a new treatment option for patients with PAH and PH-ILD
- YUTREPIA is designed to enhance deep-lung delivery with an easy-to-use device requiring low inspiratory effort
- Demonstrated tolerability and titratability in the pivotal INSPIRE study
- Liquidia will host a webcast Tuesday, May 27, 2025 at 8:30 a.m. ET to provide an update on commercial launch preparations
MORRISVILLE, N.C., May 23, 2025 (GLOBE NEWSWIRE) — Liquidia Corporation (NASDAQ: LQDA), a biopharmaceutical company developing innovative therapies for patients with rare cardiopulmonary disease, announced today that the U.S. Food and Drug Administration (FDA) has approved YUTREPIA™ (treprostinil) inhalation powder, a prostacyclin analog for adults with pulmonary arterial hypertension (PAH) and pulmonary hypertension associated with interstitial lung disease (PH-ILD) to improve exercise ability. YUTREPIA is the first and only prostacyclin dry-powder formulation enabled by Liquidia’s proprietary PRINT™ technology, which yields uniform, free-flowing particles designed to enhance deep-lung delivery via an easy-to-use, low-effort device requiring less inspiratory effort.
Dr. Roger Jeffs, Chief Executive Officer of Liquidia, said: “Today, we celebrate for the patients and physicians who will now have access to a potential best-in-class dry-powder form of treprostinil with exceptional portability, tolerability, titratability and durability. Thank you to the clinical investigation team, our steering committee, and the members of the pulmonary hypertension patient communities who helped make this day a reality. With today’s milestone, our commercial team is prepared to launch YUTREPIA and bring meaningful change to the lives of patients in need, and we look forward to speaking with physicians and patients about the unique benefits of YUTREPIA in the days and weeks ahead.”
The approval of YUTREPIA is based on findings from the Phase 3 INSPIRE trial which evaluated patients who were naïve to treprostinil, as well as those transitioning to YUTREPIA from nebulized treprostinil. YUTREPIA was shown to be safe and well-tolerated regardless of a patient’s previous exposure to treprostinil. Results from the INSPIRE study were published in the Pulmonary Circulation Journal in 2022 and the Vascular Pharmacology Journal in 2021. Please see the “Selected Safety Information” in the section entitled “About YUTREPIA™ (treprostinil) Inhalation Powder.”
Dr. Nicholas Hill, Chief Pulmonary, Critical Care & Sleep Division, Professor of Medicine at Tufts University School of Medicine and Principal Investigator on the Phase 3 INSPIRE study, said: “I am so pleased that patients with PAH and PH-ILD now have this newly introduced option for inhaled treprostinil. Having treated patients for more than six years in Liquidia’s INSPIRE and extension studies, I am confident in the safety, tolerability and dosing that YUTREPIA offers. The low-effort inhalation device used to deliver YUTREPIA may make it easier to start and maintain patients on treatment, especially those with limited inspiratory flows or lung capacity.”
Matt Granato, President and Chief Executive Officer of the Pulmonary Hypertension Association, said: “PAH and PH-ILD impact more than 105,000 patients in the U.S. alone. These patient communities and the physicians who serve them need therapies that can lead to the improvement of quality of life. We are always glad to see industry research leading to development of drugs that expand options for the patient community.”
As previously disclosed, United Therapeutics Corporation (UTHR) filed a complaint on May 9, 2025, in the U.S. District Court for the Middle District of North Carolina (Case No. 1:25-cv-00368) against Liquidia alleging infringement of U.S. Patent No. 11,357,782 (the ‘782 patent) and seeks to enjoin Liquidia from commercializing YUTREPIA to treat PAH and PH-ILD. UTHR has filed a motion for temporary restraining order and preliminary injunction to block Liquidia from commercially launching YUTREPIA. Oral argument on the motion was held on May 20, 2025. The motion remains pending with the Court.
Webcast Information
Liquidia will provide an update on YUTREPIA commercial launch preparations via a live webcast on Tuesday, May 27, 2025, at 8:30 a.m. ET. Access to the webcast will be available on the “Investors” page of Liquidia’s website at https://liquidia.com/investors/events-and-presentations. A replay and transcript of the webcast will be archived on the company’s website for at least 30 days.
About Pulmonary Arterial Hypertension (PAH)
Pulmonary arterial hypertension (PAH) is a rare, chronic, progressive disease caused by narrowing, thickening or stiffening of the pulmonary arteries that can lead to right heart failure and eventually death. Currently, an estimated 45,000 patients are diagnosed and treated in the United States. There is currently no cure for PAH, so the goals of existing treatments are to alleviate symptoms, maintain or improve functional class, delay disease progression, and improve quality of life.
About Pulmonary Hypertension Associated with Interstitial Lung Disease (PH-ILD)
Pulmonary hypertension (PH) associated with interstitial lung disease (ILD) includes a diverse collection of up to 200 different pulmonary diseases, including interstitial pulmonary fibrosis, chronic hypersensitivity pneumonitis, connective tissue disease-related ILD, and chronic pulmonary fibrosis with emphysema (CPFE) among others. Any level of PH in ILD patients is associated with poor 3-year survival. A current estimate of PH-ILD prevalence in the United States is greater than 60,000 patients, though population size in many of these underlying ILD diseases is not yet known due to factors including underdiagnosis and lack of approved treatments until March 2021, when inhaled treprostinil was first approved for this indication.
About YUTREPIA™ (treprostinil) Inhalation Powder
YUTREPIA is an inhaled dry-powder formulation of treprostinil delivered through a convenient, low-effort, palm-sized device. YUTREPIA was designed using Liquidia’s PRINT® technology, which enables the development of drug particles that are precise and uniform in size, shape and composition, and that are engineered for enhanced deposition in the lung following oral inhalation. Liquidia has completed the INSPIRE trial (NCT03399604), or Investigation of the Safety and Pharmacology of Dry Powder Inhalation of Treprostinil, an open-label, multi-center phase 3 clinical study of YUTREPIA in patients diagnosed with PAH who are naïve to inhaled treprostinil or who are transitioning from Tyvaso® (nebulized treprostinil). YUTREPIA is currently being studied in the ASCENT trial (NCT06129240), or An Open-Label ProSpective MultiCENTer Study to Evaluate Safety and Tolerability of Dry Powder Inhaled Treprostinil in PH, with the objective of informing YUTREPIA’s dosing and tolerability profile in patients with PH-ILD. YUTREPIA was previously referred to as LIQ861 in investigational studies.
INDICATION
YUTREPIA (treprostinil) inhalation powder is a prostacyclin analog indicated for the treatment of:
- Pulmonary arterial hypertension (PAH; WHO Group 1) to improve exercise ability. Studies establishing effectiveness predominately included patients with NYHA Functional Class III symptoms and etiologies of idiopathic or heritable PAH (56%) or PAH associated with connective tissue diseases (33%).
- Pulmonary hypertension associated with interstitial lung disease (PH-ILD; WHO Group 3) to improve exercise ability. The study establishing effectiveness predominately included patients with etiologies of idiopathic interstitial pneumonia (IIP) (45%) inclusive of idiopathic pulmonary fibrosis (IPF), combined pulmonary fibrosis and emphysema (CPFE) (25%), and WHO Group 3 connective tissue disease (22%).
SELECTED SAFETY INFORMATION: WARNINGS AND PRECAUTIONS
- Treprostinil is a pulmonary and systemic vasodilator. In patients with low systemic arterial pressure, treatment with Treprostinil may produce symptomatic hypotension.
- Treprostinil inhibits platelet aggregation and increases the risk of bleeding.
- Co-administration of a cytochrome P450 (CYP) 2C8 enzyme inhibitor (e.g., gemfibrozil) may increase exposure (both Cmax and AUC) to treprostinil. Co-administration of a CYP2C8 enzyme inducer (e.g., rifampin) may decrease exposure to treprostinil. Increased exposure is likely to increase adverse events associated with treprostinil administration, whereas decreased exposure is likely to reduce clinical effectiveness.
- Like other inhaled prostaglandins, YUTREPIA may cause acute bronchospasm. Patients with asthma or chronic obstructive pulmonary disease (COPD), or other bronchial hyperreactivity, are at increased risk for bronchospasm. Ensure that such patients are treated optimally for reactive airway disease prior to and during treatment.
- Most common adverse reactions with YUTREPIA (≥10%) are cough, headache, throat irritation and dizziness.
Prescribing Information and Instructions for Use for YUTREPIA (treprostinil) inhalation powder are available at YUTREPIA.com.
About Liquidia Corporation
Liquidia Corporation is a biopharmaceutical company developing innovative therapies for patients with rare cardiopulmonary disease. The company’s current focus spans the development and commercialization of products in pulmonary hypertension and other applications of its proprietary PRINT® Technology. PRINT enabled the creation of YUTREPIA™ (treprostinil) inhalation powder, a drug that has been approved for the treatment of pulmonary arterial hypertension (PAH) and pulmonary hypertension associated with interstitial lung disease (PHILD). The company is also developing L606, an investigational sustained-release formulation of treprostinil administered twice-daily with a next-generation nebulizer and currently markets generic Treprostinil Injection for the treatment of PAH. To learn more about Liquidia, please visit http://www.liquidia.com.
Tyvaso® is a registered trademark of United Therapeutics Corporation.
Cautionary Statements Regarding Forward-Looking Statements
This press release may include forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release other than statements of historical facts, including statements regarding our future results of operations and financial position, our strategic and financial initiatives, our business strategy and plans and our objectives for future operations, are forward-looking statements. Such forward-looking statements, including statements regarding clinical trials, clinical studies and other clinical work (including the funding therefor; anticipated patient enrollment, safety data, study data, trial outcomes, timing or associated costs); regulatory applications and related submission contents and timelines; our ability to successfully commercialize our products, including YUTREPIA, for which we obtain FDA or other regulatory authority approval; the acceptance by the market of our products, including YUTREPIA, and their potential pricing and/or reimbursement by third-party payors, if approved (in the case of our product candidates) and whether such acceptance is sufficient to support continued commercialization or development of our products; the successful development or commercialization of our products, including YUTREPIA; our revenue from product sales and whether or not we may become profitable in the near term, or at all; future competitive or other market factors that may adversely affect the commercial potential for YUTREPIA; and our ability to execute on our strategic or financial initiatives, involve significant risks and uncertainties and actual results could differ materially from those expressed or implied herein. Despite the approval of YUTREPIA by the FDA, it is possible that commercialization of YUTREPIA may be blocked or delayed in connection with legal proceedings that have been initiated or that may in the future be initiated, or we may be required to pay damages, including royalties, in connection with our commercial launch, as a result of these legal proceedings. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “would,” and similar expressions are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy, short-term and long-term business operations and objectives and financial needs. These forward-looking statements are subject to a number of risks discussed in our filings with the SEC, as well as a number of uncertainties and assumptions. Moreover, we operate in a very competitive and rapidly changing environment and our industry has inherent risks. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the future events discussed in this press release may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Nothing in this press release should be regarded as a representation by any person that these goals will be achieved, and we undertake no duty to update our goals or to update or alter any forward-looking statements, whether as a result of new information, future events or otherwise.
Contact Information
Investors:
Jason Adair
919.328.4350
jason.adair@liquidia.com
Media:
Patrick Wallace
919.328.4383
patrick.wallace@liquidia.com