InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the “Global AI-Enhanced Minimally Invasive Devices Market Size, Share & Trends Analysis Report By Product Type (AI-Enhanced Catheters, AI-Powered Endoscopy Systems, Robotic-Assisted Surgical Systems, Imaging and Visualization Devices, Other Devices), Application (Cardiology, Gastroenterology, Interventional Radiology, Orthopedics, Urology and Gynecology, Others), End User (Hospitals, Ambulatory Surgical Centers (ASCs), Specialty Clinics), Technology (Machine Learning, Computer Vision, Natural Language Processing (NLP), Robotics)-Market Outlook And Industry Analysis 2034”
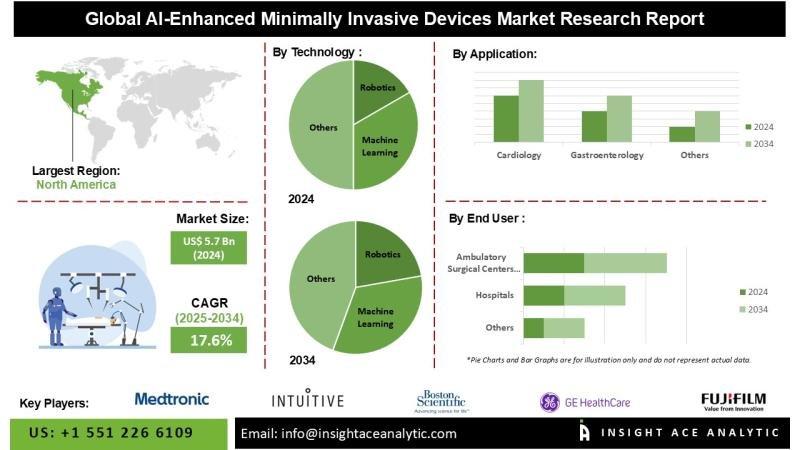
The Global AI-Enhanced Minimally Invasive Devices Market is valued at US$ 5.7 Bn in 2024 and it is expected to reach US$ 27.8 Bn by the year 2034, with a CAGR of 17.6 % during the forecast period of 2025-2034.
Get Free Access to Demo Report, Excel Pivot and ToC: https://www.insightaceanalytic.com/request-sample/2962
The incorporation of artificial intelligence (AI) into minimally invasive medical devices is revolutionizing healthcare by improving procedural accuracy, operational efficiency, and patient outcomes. AI-enabled technologies-such as robotic-assisted surgical systems, intelligent catheters, and advanced imaging platforms-utilize real-time data analytics, image recognition, and navigation algorithms to support the execution of complex interventions with minimal tissue disruption.
The global market for AI-integrated minimally invasive devices is experiencing robust growth, driven by continuous technological advancements, increasing patient preference for less invasive treatment options, and the expanding application of AI in delivering personalized healthcare solutions. These innovations offer a range of benefits, including improved surgical precision, accelerated recovery times, enhanced clinical decision-making, and reduced overall healthcare costs.
Adoption of AI-powered minimally invasive devices spans multiple medical specialties. In cardiology, these systems facilitate procedures such as stent implantation; in gastroenterology, they enhance the accuracy of advanced endoscopic diagnostics; in interventional radiology, they provide real-time procedural guidance; in orthopedics, they support spinal and joint surgeries; and in urology and gynecology, robotic-assisted platforms improve outcomes in procedures including prostatectomies and hysterectomies.
List of Prominent Players in the AI-Enhanced Minimally Invasive Devices Market:
• Medtronic Plc
• Intuitive Surgical, Inc.
• Boston Scientific Corporation
• GE HealthCare
• Fujifilm Holdings Corporation
• Stryker Corporation
• Siemens Healthineers
• Johnson & Johnson (Ethicon + Auris Health)
• Olympus Corporation
• Zimmer Biomet
• Asensus Surgical, Inc.
• CMR Surgical
• Auris Health (J&J Subsidiary)
Expert Knowledge, Just a Click Away: https://calendly.com/insightaceanalytic/30min?month=2025-04
Market Dynamics
Drivers:
The rapid advancement of artificial intelligence (AI) technologies, particularly in machine learning and computer vision, has significantly enhanced the precision, safety, and functionality of minimally invasive medical devices. These innovations have improved real-time imaging, intraoperative decision-making, and overall procedural efficacy. AI-enabled robotic-assisted systems offer superior dexterity and surgical accuracy, reducing complications and enhancing patient outcomes.
This technological progression aligns with the increasing global preference for minimally invasive procedures, which reduce patient trauma, lower complication risks, and facilitate faster recovery. Additionally, the rising prevalence of chronic conditions-including cardiovascular diseases, cancer, and diabetes, affecting approximately 129 million individuals in the United States-further fuels the demand for advanced AI-driven diagnostic and therapeutic solutions.
Challenges:
Despite their transformative potential, AI-integrated minimally invasive devices face several adoption barriers. Regulatory frameworks often lag behind rapid AI advancements, and variations in international approval processes add complexity. Technical limitations, including algorithmic bias, limited transparency, and inconsistent system performance, raise concerns regarding safety and reliability.
Ethical considerations surrounding data privacy, combined with a lack of comprehensive model validation, can undermine stakeholder confidence. Furthermore, high implementation costs, integration challenges with existing systems, and the need for ongoing training and maintenance pose additional constraints. Continuous performance monitoring and compliance with stringent regulatory standards also require significant resources from healthcare providers and device manufacturers.
Regional Trends:
North America continues to lead the AI-enabled minimally invasive medical device market, supported by advanced healthcare infrastructure, substantial expenditure, and a mature regulatory environment. The presence of leading industry players-such as Medtronic, Johnson & Johnson, and Stryker-who actively invest in innovation and product development, reinforces regional market dominance. Moreover, the growing adoption of minimally invasive procedures, driven by clinical benefits and cost-effectiveness, continues to accelerate the uptake of AI-driven solutions across healthcare institutions in the region.
Recent Developments:
• In March 2025, FUJIFILM Healthcare Americas and Us2.ai partnered to integrate AI-driven echocardiography into the LISENDO 880 cardiovascular ultrasound system. The collaboration enables full automation of echocardiogram analysis and reporting, delivering comprehensive cardiac measurements. This advancement streamlines clinical workflows and enhances diagnostic accuracy in heart disease detection.
• In March 2025, At the 2025 American College of Cardiology meeting, GE HealthCare unveiled the RevolutionTM Vibe CT system, featuring Unlimited One-Beat Cardiac imaging for high-quality scans, even in complex cases like atrial fibrillation. Integrated with AI-powered tools like ECG-less Cardiac, TrueFidelity DL, and Effortless Workflow, the system enhances diagnostic speed, accuracy, patient comfort, and clinical efficiency.
Unlock Your GTM Strategy: https://www.insightaceanalytic.com/customisation/2962
Segmentation of Trusted Platform Module Market-
By Product Type:
• AI-Enhanced Catheters
• AI-Powered Endoscopy Systems
• Robotic-Assisted Surgical Systems
• Imaging and Visualization Devices
• Other Devices
By Application:
• Cardiology
• Gastroenterology
• Interventional Radiology
• Orthopedics
• Urology and Gynecology
• Others
By End User:
• Hospitals
• Ambulatory Surgical Centers (ASCs)
• Specialty Clinics
By Technology:
• Machine Learning
• Computer Vision
• Natural Language Processing (NLP)
• Robotics
By Region-
North America-
• The US
• Canada
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• South East Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Argentina
• Mexico
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of Middle East and Africa
Read Overview Report- https://www.insightaceanalytic.com/report/ai-enhanced-minimally-invasive-devices-market/2962
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
Contact us:
InsightAce Analytic Pvt. Ltd.
Visit: http://www.insightaceanalytic.com
Tel : +1 607 400-7072
Asia: +91 79 72967118
info@insightaceanalytic.com
This release was published on openPR.