The global Regulatory Information Management (RIM) Market is projected to grow from USD 2.7 billion in 2026 to USD 7.6 billion by 2036, expanding at a strong compound annual growth rate (CAGR) of 11% during the forecast period. The sustained expansion reflects increasing regulatory complexity across life sciences industries, rising submission volumes, and accelerating adoption of centralized digital compliance platforms worldwide.
As pharmaceutical, biologics, medical device, and nutraceutical companies expand product pipelines and enter new geographies, regulatory operations have evolved into mission-critical functions. Organizations are shifting from fragmented, manual processes to integrated RIM platforms that streamline submissions, manage labeling, track lifecycle variations, and ensure consistent compliance across multiple jurisdictions.
Request For Sample Report | Customize Report | Purchase Full Report
https://www.futuremarketinsights.com/reports/sample/rep-gb-1889
Strong Demand Fundamentals Support Long-Term Market Expansion
Growth is underpinned by steady consumption in mature economies, where established infrastructure, stable policy frameworks, and consistent industrial output drive recurring compliance needs. At the same time, emerging markets are contributing a growing share of incremental demand, fueled by expanding manufacturing bases, rising exports, and increasing regulatory digitization.
RIM adoption is particularly strong in countries with advanced regulatory ecosystems and high product filing volumes, including the United States, China, Western Europe, and India. As cross-border filings increase and electronic submission mandates become universal, organizations are prioritizing enterprise-grade regulatory platforms to maintain operational efficiency and audit readiness.
Competitive dynamics are shaped by ongoing investment in platform scalability, process optimization, and cost efficiency. Vendors are strengthening delivery models, refining sourcing strategies, and enhancing integration capabilities to stabilize margins and build long-term customer partnerships.
Software Solutions Command 70% of Global Demand
By solution type, software platforms dominate the industry, accounting for approximately 70% of total global demand. These solutions manage regulatory submissions, registrations, labeling updates, and lifecycle documentation across multiple authorities.
Within the software segment, cloud-based deployments represent nearly 60% of software demand, supported by scalability, lower infrastructure requirements, and improved collaboration among geographically dispersed regulatory teams. On-premise platforms retain around 40% share, largely driven by organizations with strict internal data governance and security mandates.
Services-including consulting, implementation, training, and ongoing support-complement software adoption and remain essential for ensuring regulatory alignment and successful system integration.
Pharmaceuticals Lead Vertical Adoption
By vertical, pharmaceutical companies represent the largest share of the RIM market, contributing 45% of total demand. Frequent global filings, post-approval variations, and rigorous oversight from agencies such as the FDA, EMA, and PMDA drive sustained platform utilization.
Biopharmaceutical companies account for approximately 18% of market share, reflecting the documentation complexity associated with advanced therapies and biologics. Medical device manufacturers contribute nearly 14%, while nutraceuticals and cosmetics collectively represent 23%, supported by increasing compliance requirements and growing international trade.
The expanding complexity of global pipelines-including biosimilars, digital therapeutics, and combination products-further reinforces the need for centralized regulatory information management systems capable of handling structured data and evolving standards such as eCTD and IDMP.
Compliance Pressures and Digital Workflows Accelerate Adoption
Rising regulatory scrutiny and tighter submission timelines are compelling organizations to replace manual workflows with automated, software-driven compliance systems. RIM platforms enable real-time collaboration across regulatory, clinical, and quality teams while reducing submission errors and approval delays.
Integration of automation, analytics, and artificial intelligence is improving document processing accuracy and submission quality. As electronic submissions become mandatory across major regulatory authorities, centralized data management systems are increasingly viewed as essential infrastructure rather than optional enhancements.
Cloud platforms, in particular, are enabling faster rollouts, lower upfront capital expenditure, and improved global visibility across product lifecycles.
Regional Growth Momentum Intensifies
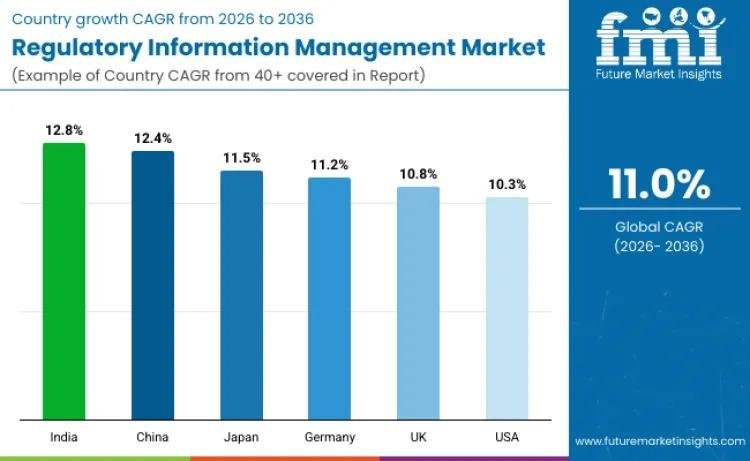
Demand patterns vary across regions, with emerging markets exhibiting the fastest expansion rates.
• India leads global growth with a CAGR of 12.8%, supported by rising pharmaceutical exports and regulatory digitization initiatives such as SUGAM.
• China follows closely at 12.4% CAGR, driven by regulatory reform under the NMPA and increasing overseas product registrations.
• Japan expands at 11.5%, reflecting PMDA alignment with international submission standards.
• Germany records 11.2% CAGR, fueled by EU digital compliance mandates and adoption of eCTD v4.0 and IDMP frameworks.
• The United Kingdom grows at 10.8%, shaped by post-Brexit regulatory divergence and parallel compliance requirements.
• The United States maintains steady expansion at 10.3%, supported by high FDA submission volumes and ongoing lifecycle management complexity.
Emerging economies are contributing a rising share of incremental demand as life sciences ecosystems mature and digital regulatory infrastructures strengthen.
Scalability Challenges and Industry Response
While growth prospects remain strong, scalability is influenced by implementation costs, integration complexity, and data security requirements. Smaller organizations often face budget constraints and limited in-house expertise, which can slow deployment.
Vendors are responding with modular SaaS platforms, managed services, and scalable pricing models that reduce entry barriers. Hybrid deployment models are gaining traction in regions with strict data sovereignty laws, balancing cloud flexibility with localized control.
Long-term industry expansion depends on successfully navigating regulatory rigor, cost control, and secure digital infrastructure deployment.
Exhaustive Market Report: A Complete Study
https://www.futuremarketinsights.com/reports/regulatory-information-management-market
Competitive Landscape Defined by Expertise and Platform Depth
The market remains moderately consolidated, with competition centered on regulatory expertise, platform breadth, and ability to manage complex global compliance workflows.
Leading providers such as Veeva Systems, ArisGlobal, PhlexGlobal, Amplexor Life Sciences, and MasterControl maintain strong positions through scalable cloud platforms, structured data management capabilities, and deep knowledge of regional regulatory frameworks.
Enterprise-focused platforms compete alongside agile solutions tailored for small and medium-sized companies. Differentiation increasingly hinges on implementation speed, integration flexibility, data security capabilities, and ongoing regulatory updates aligned with evolving global standards.
As compliance expectations intensify worldwide, demand increasingly favors vendors capable of delivering reliable regulatory outcomes while improving operational transparency, speed, and lifecycle control.
Outlook Through 2036
With global regulatory environments becoming more complex and submission volumes continuing to rise, the Regulatory Information Management market is positioned for sustained, long-term expansion. The projected growth to USD 7.6 billion by 2036 underscores the structural shift toward digital compliance infrastructure across life sciences industries.
Comprehensive analysis of segment performance, competitive positioning, country-level trends, and technology evolution is available in the full market report, offering detailed insights into one of the most strategically important segments of the global life sciences technology landscape.
Similar Industry Reports
Product Information Management Market
https://www.futuremarketinsights.com/reports/product-information-management-market
Building Information Management (BIM) Market
https://www.futuremarketinsights.com/reports/building-information-modeling-bim-software-market
Security Information and Event Management Software Market
https://www.futuremarketinsights.com/reports/security-information-and-event-management-software-market
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-845-579-5705
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
Future Market Insights, Inc. (FMI) is an ESOMAR-certified, ISO 9001:2015 market research and consulting organization, trusted by Fortune 500 clients and global enterprises. With operations in the U.S., UK, India, and Dubai, FMI provides data-backed insights and strategic intelligence across 30+ industries and 1200 markets worldwide.
This release was published on openPR.