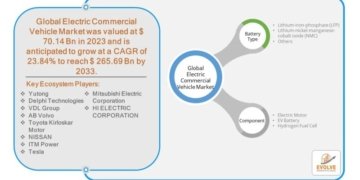
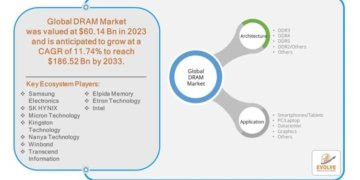
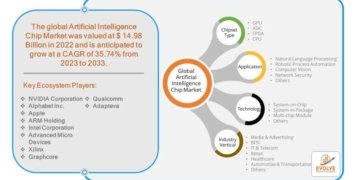
How are innovation trends and major demand drivers reshaping the catheter stabilization devices market?
The catheter stabilization devices market is being reshaped by rising clinical emphasis on patient safety, infection prevention, and cost containment across healthcare systems. Increasing hospital admissions, aging populations, and higher prevalence of chronic conditions requiring long-term catheterization are key demand drivers. Global health authorities continue to highlight the burden of hospital-acquired infections and device-related complications, prompting providers to prioritize securement solutions that reduce catheter movement, dislodgement, and secondary infections.
Innovation trends are closely aligned with value-based healthcare delivery and regulatory compliance. Hospitals and outpatient centers are shifting away from traditional adhesive tapes toward engineered stabilization devices that improve dwell time, minimize skin trauma, and support standardized care protocols. Buyer search intent increasingly reflects interest in products that combine clinical efficacy with ease of use, reduced nursing workload, and compatibility with infection control guidelines.
For C-suite executives, the market opportunity is driven by both clinical outcomes and economic impact. Catheter stabilization devices are now recognized as low-cost, high-impact tools that reduce downstream complications and length of stay. As reimbursement models and quality metrics place greater weight on preventable adverse events, demand for reliable stabilization solutions is becoming a strategic priority rather than a discretionary purchase.
Get | Download Sample Copy with TOC, Graphs & List of Figures @ https://www.verifiedmarketresearch.com/download-sample?rid=40187&utm_source=Openpr-NSL-Jan26&utm_medium=309
What key technological advancements are improving performance and compliance readiness?
Technological advancements in catheter stabilization devices focus on material innovation, skin-friendly adhesion, and universal design compatibility. Next-generation securement systems utilize breathable, hypoallergenic materials and engineered anchoring mechanisms that maintain catheter position without compromising patient comfort. These advancements support regulatory expectations for patient-centered care and reduced skin injury, particularly in vulnerable populations.
Design innovation is also improving versatility and workflow efficiency. Universal stabilization platforms capable of supporting multiple catheter types, including peripheral intravenous, central venous, and urinary catheters, are gaining adoption. Enhanced antimicrobial interfaces and moisture-resistant materials further align these devices with infection prevention strategies promoted by national healthcare quality bodies.
From a strategic standpoint, these advancements are strengthening the role of catheter stabilization devices in clinical standardization. Market intelligence teams note that suppliers offering clinically validated, easy-to-integrate solutions gain stronger traction with hospital procurement committees. Technology differentiation increasingly centers on proven outcome improvement, regulatory alignment, and seamless integration into existing care pathways.
How are changing consumption patterns influencing procurement and market strategies?
Changing consumption patterns are significantly influencing how catheter stabilization devices are evaluated and adopted. Healthcare providers are moving toward evidence-based purchasing, prioritizing products that demonstrate measurable reductions in complications and nursing intervention time. The expansion of ambulatory care, home healthcare, and long-term care facilities is also driving demand for stabilization devices that support extended use outside acute hospital settings.
In parallel, workforce constraints and increased emphasis on protocol-driven care are accelerating adoption of easy-to-apply, training-efficient stabilization systems. Procurement decisions are increasingly centralized, with group purchasing organizations and integrated delivery networks favoring scalable solutions that ensure consistency across multiple care settings. This trend is reinforced by regulatory scrutiny around infection prevention and patient safety reporting.
For executives and product strategists, these shifts highlight the importance of outcome-focused positioning and clinical education. Success in the catheter stabilization devices market depends on aligning product design with evolving care models, regulatory expectations, and total cost-of-care considerations. Companies that anticipate these consumption patterns and demonstrate clear clinical and economic value are better positioned for sustainable growth and long-term market leadership.
The competitive landscape of a market explains strategies incorporated by key players of the Catheter Stabilization Devices Market. Key developments and shifts in management in recent years by players have been explained through company profiling. This helps readers to understand the trends that will accelerate the growth of the Catheter Stabilization Devices Market. It also includes investment strategies, marketing strategies, and product development plans adopted by major players of the Catheter Stabilization Devices Market. The market forecast will help readers make better investments.
The report covers extensive analysis of the key market players in the market, along with their business overview, expansion plans, and strategies. The key players studied in the report include:
ConvaTec
Inc.
Baxter International Inc.
TIDI Products LLC
3M Company
Smiths Medical.
Centurion Medical Products
B Braun Melsungen AG
C.R. Bard Inc
Merit Medical Systems
Inc.
Cardinal Health.
Catheter Stabilization Devices Market Segmentation
Catheter Stabilization Devices Market, By Application
• Cardiovascular Procedures
• Respiratory Procedures
• General Surgery
• Other Applications
Catheter Stabilization Devices Market, By Product Type
• Chest Drainage Tube Securement Devices
• Peripheral Securement Devices
• Arterial Securement Devices
• Others
Catheter Stabilization Devices Market, By End User
• Hospitals
• Home Care Settings
• Emergency Clinics
Catheter Stabilization Devices Market By Geography
• North America
• Europe
• Asia Pacific
• Latin America
• Middle East and Africa
The comprehensive segmental analysis offered in the report digs deep into important types and application segments of the Catheter Stabilization Devices Market. It shows how leading segments are attracting growth in the Catheter Stabilization Devices Market. Moreover, it includes accurate estimations of the market share, CAGR, and market size of all segments studied in the report.
Get Discount On The Purchase Of This Report @ https://www.verifiedmarketresearch.com/ask-for-discount?rid=40187&utm_source=Openpr-NSL-Jan26&utm_medium=309
The regional segmentation study is one of the best offerings of the report that explains why some regions are taking the lead in the Catheter Stabilization Devices Market while others are making a low contribution to the global market growth. Each regional market is comprehensively researched in the report with accurate predictions about its future growth potential, market share, market size, and market growth rate.
Geographic Segment Covered in the Report:
• North America (USA and Canada)
• Europe (UK, Germany, France and the rest of Europe)
• Asia Pacific (China, Japan, India, and the rest of the Asia Pacific region)
• Latin America (Brazil, Mexico, and the rest of Latin America)
• Middle East and Africa (GCC and rest of the Middle East and Africa)
Key questions answered in the report:
• What is the growth potential of the Catheter Stabilization Devices Market?
• Which product segment will take the lion’s share?
• Which regional market will emerge as a pioneer in the years to come?
• Which application segment will experience strong growth?
• What growth opportunities might arise in the Welding industry in the years to come?
• What are the most significant challenges that the Catheter Stabilization Devices Market could face in the future?
• Who are the leading companies on the Catheter Stabilization Devices Market?
• What are the main trends that are positively impacting the growth of the market?
• What growth strategies are the players considering to stay in the Catheter Stabilization Devices Market?
For More Information or Query or Customization Before Buying, Visit @ https://www.verifiedmarketresearch.com/product/catheter-stabilization-devices-market/
Contact us:
Mr. Edwyne Fernandes
US: +1 (650)-781-4080
US Toll-Free: +1 (800)-782-1768
About Us: Verified Market Research
Verified Market Research is a leading Global Research and Consulting firm servicing over 5000+ global clients. We provide advanced analytical research solutions while offering information-enriched research studies.
We also offer insights into strategic and growth analyses and data necessary to achieve corporate goals and critical revenue decisions.
Our 250 Analysts and SMEs offer a high level of expertise in data collection and governance using industrial techniques to collect and analyze data on more than 25,000 high-impact and niche markets. Our analysts are trained to combine modern data collection techniques, superior research methodology, expertise, and years of collective experience to produce informative and accurate research.
This release was published on openPR.