The electronic trial master file (eTMF) system market value in 2024 is estimated to be around USD 1.7 billion, as per a recent report. Clinical trial conduct has increased, and eTMF software developers are supporting this trend by offering scalable and globally accessible eTMF hosting.
The electronic trial master file (eTMF) system market is evolving rapidly as clinical trial sponsors, contract research organizations (CROs), and regulatory agencies demand greater transparency, speed, and compliance in trial documentation. With increasing complexity in global clinical trials and tightening regulations, eTMF systems have become indispensable tools in modern clinical trial management.
Get Ahead with Our Report: Request Your Sample Now!
https://www.futuremarketinsights.com/reports/sample/rep-gb-1650
Modernizing Document Management in Clinical Trials
Traditionally, clinical trial documentation relied on vast amounts of paper-based records, making access, sharing, and auditing slow and inefficient. eTMF systems provide a centralized digital platform to manage, organize, and track essential trial documents in real time. These systems enable stakeholders across geographies to collaborate seamlessly while maintaining version control, audit trails, and standardized workflows.
Accelerating Regulatory Compliance and Inspection Readiness
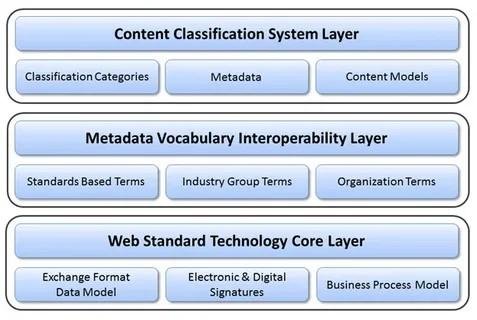
As global regulatory frameworks become more stringent, eTMF solutions are helping trial sponsors remain compliant with requirements from agencies such as the FDA, EMA, and MHRA. eTMF platforms are designed to support the TMF Reference Model, ensuring consistent classification and organization of documents. Built-in audit readiness features such as automated alerts, role-based access, and comprehensive activity logs simplify compliance and facilitate quicker inspections.
Enhancing Trial Visibility and Oversight
eTMF systems provide real-time insights into document status, completion rates, and missing elements, which are critical for trial oversight. Dashboards and analytics tools enable trial managers to track progress, ensure completeness, and proactively identify bottlenecks. This enhanced visibility contributes to improved trial efficiency and better decision-making throughout the study lifecycle.
Thorough Market Evaluation: Full Report
https://www.futuremarketinsights.com/reports/etmf-market
Seamless Collaboration Across Stakeholders
Clinical trials often involve a diverse range of stakeholders including sponsors, CROs, investigators, site staff, and regulators. eTMF systems offer secure, cloud-based access to trial documents from any location, enabling collaborative document creation, review, and approval. These platforms reduce delays associated with manual file transfers and miscommunication, promoting smoother coordination across trial teams.
Integration with Clinical Trial Management Systems (CTMS) and EDC Platforms
To streamline trial workflows, eTMF systems are increasingly being integrated with CTMS, electronic data capture (EDC), and regulatory information management systems. This integration reduces redundancy, improves data accuracy, and allows for automated population of essential documents. By linking key clinical systems, organizations can ensure end-to-end visibility and harmonization across trial operations.
Mitigating Risk and Ensuring Data Integrity
Risk-based monitoring has become a standard practice in clinical trials. eTMF solutions support this by enabling predefined workflows, automated compliance checks, and timely alerts for deviations or missing files. These capabilities help reduce errors, prevent regulatory findings, and ensure high data quality throughout the study.
Scalability and Flexibility for Diverse Trial Needs
Whether managing a single-site study or a multi-country, multi-phase trial, eTMF systems are built to scale. Vendors offer customizable templates, flexible deployment options, and language localization to meet the unique needs of each trial. As trials become more complex and global, the ability to configure the system to match specific regulatory and operational requirements is becoming a key advantage.
Driving Adoption Through User Experience and AI
User-friendly interfaces, drag-and-drop functionalities, and intuitive dashboards are accelerating the adoption of eTMF systems among clinical trial professionals. Additionally, advancements in artificial intelligence are enabling features such as automated document classification, metadata tagging, and predictive compliance tracking. These innovations are reducing administrative burdens and increasing user engagement.
Future Outlook
The electronic trial master file system market is expected to witness continued growth as the clinical research industry embraces digital transformation. As sponsors and CROs look to improve efficiency, ensure compliance, and reduce costs, eTMF platforms will serve as critical enablers of faster, more transparent, and higher-quality clinical trials. With growing integration, intelligent automation, and a focus on user-centric design, eTMF systems are reshaping how clinical trials are documented and managed in the digital age.
Catapult Your Strategy: Secure Key Insights with Our Report Checkout!
https://www.futuremarketinsights.com/checkout/1650
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-845-579-5705
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of 400 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
This release was published on openPR.