According to a new study by DataHorizzon Research, the calcineurin inhibitor drug market is projected to grow at a CAGR of 6.7% from 2025 to 2033. This clinically grounded and structurally reinforced growth trajectory is being driven by rising global organ transplantation volumes, expanding therapeutic application of calcineurin inhibitors across autoimmune and inflammatory dermatological conditions, and growing patient access to transplant medicine infrastructure across emerging healthcare economies. The calcineurin inhibitor drug market occupies an irreplaceable position within transplant immunosuppression pharmacotherapy – providing tacrolimus, cyclosporine, and voclosporin-based treatment regimens that prevent allograft rejection and manage T-cell mediated autoimmune disease activity across a broad spectrum of clinical indications. As transplant program capacity expands across Asia-Pacific and Latin America, and as next-generation calcineurin inhibitor formulations with improved bioavailability and reduced nephrotoxicity profiles gain regulatory approval and clinical adoption, the calcineurin inhibitor drug market is evolving beyond established generic commodity dynamics into a domain of meaningful pharmaceutical innovation. Market size projections and industry growth analysis confirm structurally anchored demand through the full forecast horizon.
Calcineurin Inhibitor Drug Market Key Growth Drivers and Demand Factors
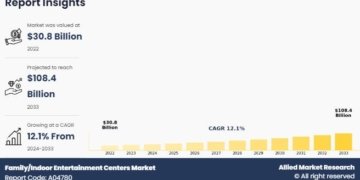
The global calcineurin inhibitor drug market was valued at USD 4.8 billion in 2024 and is projected to reach USD 9.2 billion by 2033, growing at a CAGR of 6.7% during the forecast period (2025-2033).
The calcineurin inhibitor drug market is advancing on a well-supported convergence of transplant medicine expansion, autoimmune disease prevalence growth, and pharmaceutical innovation investment that collectively generate diversified and durable demand across branded, generic, and next-generation formulation segments. The most foundational structural driver is the sustained global increase in solid organ transplantation volumes – kidney, liver, heart, and lung transplant programs are expanding capacity across both established and emerging healthcare systems, with every new transplant recipient representing a lifelong calcineurin inhibitor drug market prescription commitment that anchors long-duration revenue streams for branded and generic drug manufacturers.
Autoimmune and inflammatory dermatological indication expansion is creating meaningful demand diversification within the calcineurin inhibitor drug market beyond transplant immunosuppression. Topical tacrolimus and pimecrolimus formulations for atopic dermatitis management, voclosporin approval for lupus nephritis treatment, and ongoing clinical investigation of calcineurin inhibitor applications in psoriasis, uveitis, and inflammatory bowel disease are collectively expanding the addressable patient population of the calcineurin inhibitor drug market well beyond the transplant recipient base.
Pharmaceutical investment in next-generation calcineurin inhibitor formulations with enhanced pharmacokinetic profiles – including extended-release tacrolimus formulations designed to reduce peak-trough fluctuation and improve medication adherence – is generating premium segment revenue growth within the calcineurin inhibitor drug market. LSI-aligned demand categories including tacrolimus immunosuppressive therapy, cyclosporine modified formulations, voclosporin lupus nephritis treatment, topical calcineurin inhibitor dermatology applications, and transplant rejection prophylaxis protocols are all registering consistent prescription growth, reinforcing the calcineurin inhibitor drug market forecast through 2033.
Get a free sample report: https://datahorizzonresearch.com/request-sample-pdf/calcineurin-inhibitor-drug-market-46801
Why Choose Our Calcineurin Inhibitor Drug Market Research Report
Our research report on the calcineurin inhibitor drug market is purpose-built for pharmaceutical executives, transplant medicine specialty investors, generic drug portfolio managers, and healthcare market access strategists who require intelligence grounded in real clinical prescription dynamics, regulatory approval trajectory data, and competitive formulation differentiation factors. The report integrates primary research from transplant nephrology and hepatology clinical programs, pharmaceutical procurement administrators, hospital formulary decision-makers, and specialty pharmacy distribution channel operators – ensuring that every analytical layer reflects authentic calcineurin inhibitor drug market demand patterns and prescribing behavior realities.
The competitive landscape section delivers granular profiling of both globally operating branded pharmaceutical companies holding next-generation calcineurin inhibitor drug market positions and high-volume generic manufacturers competing on bioequivalence, supply chain reliability, and institutional contract pricing across commodity tacrolimus and cyclosporine segments. Revenue forecasting integrates transplant program volume growth projections, branded-to-generic substitution rate modeling, pipeline approval probability assessment, and indication expansion clinical milestone tracking – producing projections with authentic pharmaceutical market grounding.
Segmentation spans drug type, formulation, indication, distribution channel, end-user setting, and regional performance – equipping buyers of this report with the layered intelligence needed to evaluate portfolio development investment, identify licensing and acquisition opportunities, and benchmark competitive positioning across the global calcineurin inhibitor drug market. This report represents the most comprehensive and decision-ready market intelligence available for this specialized therapeutic sector.
Top Reasons to Invest in the Calcineurin Inhibitor Drug Market Report
• Transplant volume growth demand alignment: The calcineurin inhibitor drug market is directly correlated with global organ transplantation program expansion – this report maps active transplant program capacity growth initiatives across key geographies, enabling pharmaceutical manufacturers and investors to quantify the prescription volume pipeline with clinical program grounding.
• Next-generation formulation opportunity assessment: Evaluate which extended-release, reduced-nephrotoxicity, and improved bioavailability calcineurin inhibitor drug market formulation innovations are generating meaningful clinical adoption advantages over standard-of-care options – and where pipeline development investment offers defensible intellectual property and premium pricing potential.
• Indication expansion revenue modeling: Understand how voclosporin’s lupus nephritis approval, expanding topical calcineurin inhibitor dermatology indications, and ongoing clinical investigation across new autoimmune applications are diversifying and expanding the total addressable revenue of the calcineurin inhibitor drug market beyond transplant immunosuppression.
• Generic competition landscape navigation: Identify where commodity tacrolimus and cyclosporine segments within the calcineurin inhibitor drug market are experiencing margin compression from generic entry versus where formulation complexity, manufacturing barrier, or hospital formulary preference dynamics sustain branded pricing premiums.
• Emerging market transplant infrastructure pipeline: Use regional transplant program development data from the calcineurin inhibitor drug market forecast to identify which emerging healthcare economies are investing in transplant surgical capacity and immunosuppression pharmacy infrastructure – and where first-mover formulary positioning generates durable market share advantages
• M&A and licensing opportunity identification: Leverage detailed company profiling and pipeline analysis to evaluate acquisition targets, co-development licensing candidates, and specialty distribution partnership opportunities across the global calcineurin inhibitor drug market ecosystem.
Calcineurin Inhibitor Drug Market Challenges, Risks, and Barriers
Despite clinically anchored demand, the calcineurin inhibitor drug market faces substantive commercial and clinical constraints. Nephrotoxicity, neurotoxicity, and metabolic side effect profiles associated with chronic calcineurin inhibitor exposure create clinical pressure toward minimization protocols and calcineurin inhibitor-sparing immunosuppression regimens that limit long-term dose escalation and increase substitution risk. Generic competition across tacrolimus and cyclosporine segments is creating sustained pricing pressure that erodes branded product margins in established markets. Supply chain concentration risk for active pharmaceutical ingredient sourcing creates production continuity vulnerability. Narrow therapeutic index characteristics require intensive therapeutic drug monitoring infrastructure that complicates access in resource-limited healthcare settings. Additionally, emerging competing immunosuppressive drug classes including belatacept and JAK inhibitors present long-term substitution risk for certain calcineurin inhibitor drug market indications.
Top 10 Companies in the Calcineurin Inhibitor Drug Market
• Astellas Pharma Inc.
• Novartis AG
• Aurinia Pharmaceuticals Inc.
• Pfizer Inc.
• Mylan N.V. (Viatris Inc.)
• Sandoz International GmbH (Novartis)
• Teva Pharmaceutical Industries Ltd.
• Dr. Reddy’s Laboratories Ltd.
• Veloxis Pharmaceuticals A/S
• Hikma Pharmaceuticals plc
Market Segmentation
By Drug Type
o Tacrolimus (Immediate-release, Extended-release)
o Cyclosporine (Oral, Injectable)
o Pimecrolimus (Topical formulations)
By Application
o Organ Transplantation (Kidney, Liver, Heart, Lung)
o Dermatitis (Atopic dermatitis, Contact dermatitis)
o Autoimmune Disorders (Rheumatoid arthritis, Lupus)
By Distribution Channel
o Hospital Pharmacies
o Retail Pharmacies
o Online Pharmacies
By Patient Type
o Adult
o Pediatric
o Geriatric
By Formulation
o Oral
o Injectable
o Topical
By End User
o Hospitals
o Specialty Clinics
o Transplant Centers
o Research Institutes
By Region:
o North America
o Europe
o Latin America
o Asia Pacific
o Middle East and Africa
Recent Developments in the Calcineurin Inhibitor Drug Market
• In early 2025, a leading calcineurin inhibitor drug market manufacturer received regulatory approval in the European Union for an extended-release once-daily tacrolimus formulation demonstrating statistically equivalent immunosuppressive efficacy with significantly improved medication adherence outcomes compared to twice-daily standard tacrolimus regimens in a Phase III kidney transplant recipient trial – establishing a meaningful clinical differentiation platform within the branded calcineurin inhibitor drug market segment.
• A prominent specialty pharmaceutical company active in the calcineurin inhibitor drug market announced positive Phase II clinical trial results in late 2024 for a novel low-dose voclosporin combination regimen in IgA nephropathy – expanding the potential clinical application scope of voclosporin beyond its established lupus nephritis indication and creating a meaningful near-term pipeline catalyst for the calcineurin inhibitor drug market.
• A significant licensing agreement was formalized in 2025 between a global pharmaceutical company and a specialty calcineurin inhibitor drug market developer, granting co-commercialization rights for a novel topical tacrolimus nanoparticle formulation for moderate-to-severe atopic dermatitis across North American and European markets – combining development expertise with established dermatology sales infrastructure.
• A leading generic pharmaceutical company within the calcineurin inhibitor drug market completed the acquisition of a specialty transplant immunosuppression drug manufacturer in late 2024, consolidating its oral tacrolimus and cyclosporine portfolio while gaining access to proprietary extended-release formulation technology assets and established transplant center formulary relationships.
• Several calcineurin inhibitor drug market manufacturers announced active pharmaceutical ingredient supply chain diversification programs in 2024-2025, qualifying multiple secondary API sourcing partners and establishing regional safety stock inventory protocols to address the supply chain concentration risk concerns that had created periodic product availability disruptions across global transplant pharmacy networks.
• A major Asian pharmaceutical group expanded its calcineurin inhibitor drug market manufacturing capacity in 2025 by commissioning a new GMP-certified tacrolimus API and finished dosage form production facility, targeting growing domestic transplant program demand across China, South Korea, and Southeast Asia alongside export supply commitments to regulated markets in Europe and North America.
Calcineurin Inhibitor Drug Market Regional Performance & Geographic Expansion
North America dominates the calcineurin inhibitor drug market in per-patient drug expenditure and branded formulation revenue concentration, with the United States anchoring global industry size through its high-volume solid organ transplant program infrastructure, comprehensive specialty pharmacy reimbursement frameworks, and active adoption of next-generation extended-release and voclosporin formulations. Europe follows with structured, guideline-driven demand – Germany, France, the United Kingdom, and Italy generating consistent calcineurin inhibitor drug market prescription volumes through comprehensive transplant program networks and well-established immunosuppression protocol adoption. Asia-Pacific is the fastest-growing regional segment, with China, Japan, South Korea, and India registering accelerating demand driven by transplant program capacity expansion and improving patient access to standard-of-care immunosuppressive therapy. Latin America is progressing through public health system transplant infrastructure development in Brazil and Mexico. The Middle East & Africa region is emerging through transplant center development in Saudi Arabia, the UAE, and South Africa.
How Calcineurin Inhibitor Drug Market Insights Drive ROI Growth
Pharmaceutical manufacturers, specialty drug investors, and healthcare market access strategists that operationalize structured intelligence from the calcineurin inhibitor drug market consistently achieve superior commercial outcomes across portfolio development, geographic market entry, and competitive positioning dimensions. Precise indication-level demand forecasting by drug type, formulation, and geographic market enables pipeline investment decisions aligned with genuine prescription volume growth trajectories within the calcineurin inhibitor drug market. Competitive benchmarking drawn from the calcineurin inhibitor drug market forecast reveals formulation differentiation gaps – whether in extended-release bioavailability improvement, nephrotoxicity reduction clinical evidence, or pediatric dosing form availability – that represent actionable product development investment priorities for manufacturers seeking to sustain premium positioning against generic erosion pressure. Forecast leverage allows business development and commercial planning teams to model revenue scenarios against branded patent protection timelines, indication expansion approval probability estimates, and emerging market transplant program ramp trajectories. Organizations that embed calcineurin inhibitor drug market intelligence into their strategic planning consistently report stronger pipeline licensing negotiation outcomes, more defensible portfolio investment presentations to institutional investors, and higher success rates in hospital formulary inclusion programs.
Sustainability & Regulatory Outlook
Sustainability considerations are gaining meaningful relevance within the calcineurin inhibitor drug market, primarily through the growing pharmaceutical industry commitment to sustainable active pharmaceutical ingredient manufacturing processes and supply chain environmental impact reduction. Tacrolimus and cyclosporine API synthesis involves complex fermentation and chemical synthesis processes with significant solvent, energy, and water consumption footprints – areas where leading manufacturers are investing in green chemistry process optimization, waste reduction engineering, and renewable energy procurement to reduce manufacturing environmental impact and meet increasingly stringent pharmaceutical supplier ESG assessment criteria imposed by major health system procurement organizations. Manufacturers that demonstrate credible environmental management system certification and quantified manufacturing sustainability improvement programs are gaining procurement preference in sustainability-conscious health system formulary evaluation processes within the calcineurin inhibitor drug market.
The regulatory landscape governing the calcineurin inhibitor drug market is comprehensive, scientifically rigorous, and continuously evolving across multiple dimensions. FDA bioequivalence standards for narrow therapeutic index drugs – of which tacrolimus is a designated member – impose the most stringent generic substitution qualification requirements in the pharmaceutical regulatory framework, requiring sponsors to demonstrate not only average bioequivalence but also individual subject bioequivalence across population variability parameters. This regulatory rigor creates higher generic market entry barriers for tacrolimus than for standard bioequivalence pathway drugs, preserving branded formulation differentiation opportunities within the calcineurin inhibitor drug market for manufacturers who invest in clinical bioequivalence evidence generation.
European Medicines Agency regulatory pathway evolution for next-generation calcineurin inhibitor formulations, including guidance on extended-release tacrolimus bioequivalence assessment and novel formulation pediatric investigation plan requirements, is shaping development program design and timeline planning for companies advancing pipeline calcineurin inhibitor drug market assets. Post-marketing pharmacovigilance requirements for chronic immunosuppression therapies – including mandatory long-term safety registry participation and periodic benefit-risk evaluation reporting – create ongoing regulatory compliance investment obligations for all calcineurin inhibitor drug market participants. The convergence of narrow therapeutic index regulatory rigor, indication expansion approval pathway complexity, and emerging market pharmaceutical registration requirements is creating a multi-layer regulatory environment that rewards sustained scientific investment and regulatory expertise within the calcineurin inhibitor drug market through the forecast period.
Key Questions Answered in the Report
1. What is the projected revenue forecast for the calcineurin inhibitor drug market through 2033, and how do transplant volume growth, generic price erosion dynamics, and next-generation formulation adoption rates affect branded versus generic revenue scenario modeling?
2. Which region will dominate the calcineurin inhibitor drug market over the forecast period, and what transplant program infrastructure investment, reimbursement policy framework, and emerging market healthcare access factors underpin that regional leadership position?
3. What are the highest-margin drug categories, formulation types, and therapeutic indication segments within the calcineurin inhibitor drug market, and how are extended-release innovation and indication diversification driving premium pricing sustainability against generic competition?
4. Who are the emerging challengers gaining competitive ground in the calcineurin inhibitor drug market, and what pipeline innovation, geographic market entry, or formulation technology strategies are enabling their market share advancement?
5. How are narrow therapeutic index bioequivalence regulatory standards, post-marketing pharmacovigilance requirements, and emerging market pharmaceutical registration frameworks shaping drug development investment priorities and competitive barrier dynamics within the calcineurin inhibitor drug market?
6. What M&A activity, licensing deal structures, and API supply chain investment trends are most likely to reshape the calcineurin inhibitor drug market competitive landscape over the next five to eight years?
Contact:
Ajay N
Ph: +1-970-633-3460
Latest Reports:
Uterine Positioning System (UPS) Market: https://datahorizzonresearch.com/uterine-positioning-systemups-market-23421
Air Cooled Transformer Market: https://datahorizzonresearch.com/air-cooled-transformer-market-24097
Industrial Occupational Safety Footwear Market: https://datahorizzonresearch.com/industrial-occupational-safety-footwear-market-24773
Utility Pump Market: https://datahorizzonresearch.com/utility-pump-market-25449
Company Name: DataHorizzon Research
Address: North Mason Street, Fort Collins,
Colorado, United States.
Mail: sales@datahorizzonresearch.com
DataHorizzon is a market research and advisory company that assists organizations across the globe in formulating growth strategies for changing business dynamics. Its offerings include consulting services across enterprises and business insights to make actionable decisions. DHR’s comprehensive research methodology for predicting long-term and sustainable trends in the market facilitates complex decisions for organizations.
This release was published on openPR.