Introduction
The ePRO, E-patient Diaries, and eCOA market has experienced significant growth due to the rising adoption of digital health technologies. Electronic Patient-Reported Outcomes (ePRO), Electronic Clinical Outcome Assessments (eCOA), and E-patient Diaries play a crucial role in modern clinical trials, improving data accuracy, patient engagement, and regulatory compliance. These tools streamline clinical research processes by replacing traditional paper-based methods with digital solutions, ensuring more efficient data collection and real-time monitoring.
This report provides an in-depth analysis of the ePRO, E-patient Diaries, and eCOA market, including key growth drivers, challenges, market trends, technological advancements, and regional insights.
Get a Sample PDF Brochure of the Report (Use Corporate Email ID for a Quick Response): http://www.persistencemarketresearch.com/samples/11506
Market Projections and Forecast
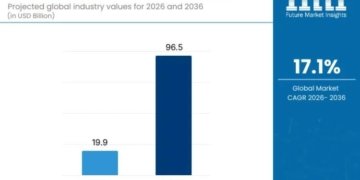
According to market projections, the global ePRO, E-patient Diaries, and eCOA market is set to grow from an estimated USD 18.2 billion in 2024 to USD 50.5 billion by 2031, reflecting a compound annual growth rate (CAGR) of 15.70% during the forecast period.
The growing emphasis on remote patient monitoring, increasing clinical trial complexity, and stringent regulatory requirements are fueling market expansion. North America, with its advanced healthcare infrastructure and strong regulatory framework, is expected to dominate the market, followed by Europe and Asia-Pacific.
Market Dynamics
Drivers of Market Growth
Rising Adoption of Digital Health Solutions:
The increasing shift toward digitalization in healthcare is a major driver for the adoption of ePRO, eCOA, and E-patient Diaries. These solutions enhance patient engagement, improve compliance, and facilitate real-time monitoring, leading to better clinical trial outcomes.
Regulatory Compliance and Data Accuracy:
Regulatory bodies such as the FDA and EMA emphasize the importance of accurate patient-reported data in clinical trials. ePRO and eCOA solutions help in maintaining data integrity, reducing errors, and ensuring compliance with regulatory standards.
Increased Use of Decentralized and Virtual Trials:
The rise of decentralized and virtual clinical trials has significantly increased the demand for ePRO and eCOA solutions. These tools allow patients to participate in trials remotely, reducing the burden on healthcare facilities and improving participant retention rates.
Advancements in AI and Analytics:
The integration of AI-driven analytics in ePRO and eCOA solutions enables real-time data insights, predictive analytics, and enhanced patient adherence monitoring. These advancements are helping pharmaceutical companies and CROs optimize clinical trial processes.
Challenges in the Market
Data Privacy and Security Concerns:
As digital health solutions handle sensitive patient data, concerns regarding data privacy and cybersecurity threats remain key challenges. Ensuring compliance with HIPAA, GDPR, and other data protection regulations is crucial for market players.
High Implementation Costs:
The initial costs associated with implementing ePRO and eCOA solutions, including software development, integration, and training, can be a barrier for small and mid-sized clinical research organizations (CROs).
Lack of Digital Literacy Among Patients:
While digital solutions improve clinical trial efficiency, some patient populations, particularly elderly and rural communities, may face challenges in adapting to new technologies. Efforts to enhance user-friendly interfaces and provide training can help overcome this barrier.
Market Trends and Technological Innovations
Integration of AI and Machine Learning:
AI-powered analytics and machine learning algorithms are being integrated into ePRO and eCOA platforms to enhance data accuracy, detect anomalies, and provide real-time patient insights.
Wearable Devices and Mobile Health Applications:
The increasing adoption of wearable devices and mobile health applications is enabling real-time data collection and remote patient monitoring, further driving the growth of eCOA solutions.
Cloud-Based eCOA Solutions:
Cloud-based platforms are gaining traction due to their scalability, ease of access, and ability to integrate with other healthcare systems such as EHRs and telemedicine solutions.
Blockchain for Data Security:
The implementation of blockchain technology is emerging as a solution to enhance data security, ensure transparency, and prevent data manipulation in clinical trials.
ePRO, E-patient Diaries and eCOA Market Segmentation
By Type:
ePRO Solutions
eCOA Solutions
E-patient Diaries
Wearable-integrated Solutions
By End-Use Industry:
Pharmaceuticals & Biotechnology
Contract Research Organizations (CROs)
Hospitals & Healthcare Providers
Academic & Research Institutions
By Deployment:
Cloud-Based
On-Premise
By Application:
Clinical Trials
Remote Patient Monitoring
Disease Management
Regional Analysis
North America
North America is expected to dominate the ePRO, E-patient Diaries, and eCOA market due to its strong presence of pharmaceutical companies, regulatory compliance standards, and well-established digital health infrastructure. The increasing number of clinical trials conducted in the U.S. is also contributing to market expansion.
Europe
Europe follows closely, driven by the increasing adoption of eHealth solutions, government initiatives supporting digital healthcare, and growing collaborations between pharmaceutical companies and technology providers. The U.K., Germany, and France are among the leading countries in this market.
Asia-Pacific
The Asia-Pacific region is expected to witness the highest CAGR during the forecast period due to rising clinical trial activities, expanding healthcare infrastructure, and growing awareness about digital health solutions in countries such as China, India, and Japan.
Key Companies Profiled in the Report
Medidata Solutions (Dassault Systèmes)
ICON plc
Parexel International Corporation
CRF Health (Signant Health)
ERT (Clario)
Oracle Corporation
ArisGlobal
Medable, Inc.
eClinicalWorks
Covance Inc. (Labcorp Drug Development)
IBM Watson Health
Veeva Systems
Future Outlook
The future of the ePRO, E-patient Diaries, and eCOA market looks promising, with substantial growth expected in both developed and emerging regions. As the demand for real-time patient data, decentralized trials, and digital healthcare solutions continues to rise, market players are investing in AI-driven analytics, cloud-based solutions, and blockchain technology to enhance security and efficiency.
Furthermore, the increasing focus on regulatory compliance, patient-centric approaches, and interoperability with other healthcare IT solutions will drive further innovation in the industry. Companies are expected to form strategic partnerships and collaborations to enhance their market presence and offer integrated digital health solutions.
Conclusion
The ePRO, E-patient Diaries, and eCOA market is undergoing rapid transformation, driven by technological advancements, regulatory mandates, and the growing need for efficient clinical trial management. While challenges such as data security and high implementation costs persist, the market’s potential for growth remains significant.
By leveraging AI, cloud computing, and mobile health technologies, companies can enhance patient engagement, streamline data collection, and improve clinical trial outcomes. As the industry continues to evolve, ePRO and eCOA solutions will play a vital role in shaping the future of digital healthcare and clinical research.
Explore the Latest Trending “Exclusive Article” @
• https://prnewssync.medium.com/bio-based-polyurethane-market-analysis-by-application-segment-2bf326969906
• https://webrankmedia.wordpress.com/2025/02/13/bio-based-polyurethane-market-emerging-innovations-and-technologies/
• https://apsnewsmedia.blogspot.com/2025/02/bio-based-polyurethane-market.html
• https://www.manchesterprofessionals.co.uk/article/marketing-pr/82603/bio-based-polyurethane-market-regional-insights-and-opportunities
• https://vocal.media/stories/bio-based-polyurethane-market-raw-material-sources-and-supply-chain
Contact Us:
Persistence Market Research
G04 Golden Mile House, Clayponds Lane
Brentford, London, TW8 0GU UK
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies’ clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we’ve built over the years.
This release was published on openPR.